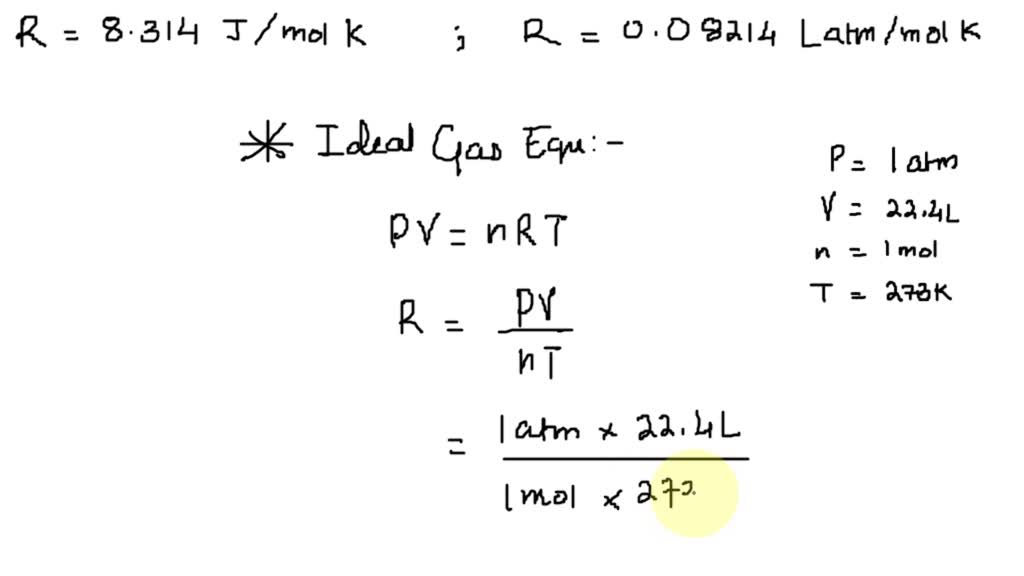
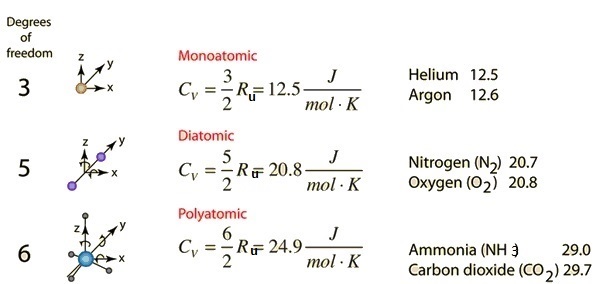
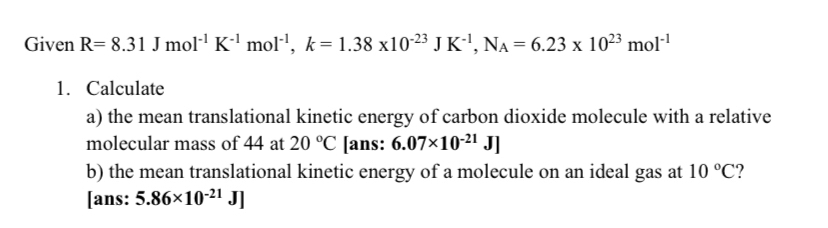36.) Calculate the rms speed of an ideal diatomic gas having molecular weight 32 gm/mol at Oc If the specific heats at constant pressure and volume are respectively 29.1 J mol1 K
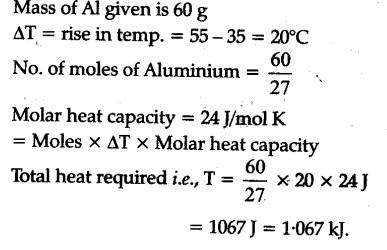
Calculate the number of kj necessary to raise the temperature of 60 g of Aluminium from 35 to 55°C. Molar heat capacity of A1 is 24 J ${{mol }^{-1}}$${{K}^{-1}}$ - CBSE Class 11