
Given, 2CO(g) C(s) + CO2(g); Kp1 = 10^-14atm^-1 at 1120 K CO(g) + Cl2(g) COCl2(g); Kp2 = 6 × 10^-3 atm^-1 The value of Kc for the following reaction at 1120 K

For the reaction, c(s)+co2(g)⇌2co(g), the partial pressures of co2 and co are 2.0 and 4.0 atm respectively - Brainly.in

For the reaction: C (s)+ CO2 (g) 2CO (g) the partial pressure of CO2 and CO are 2 and 4 atm respectively at equilibrium. Then equilibrium costant for the reaction is -







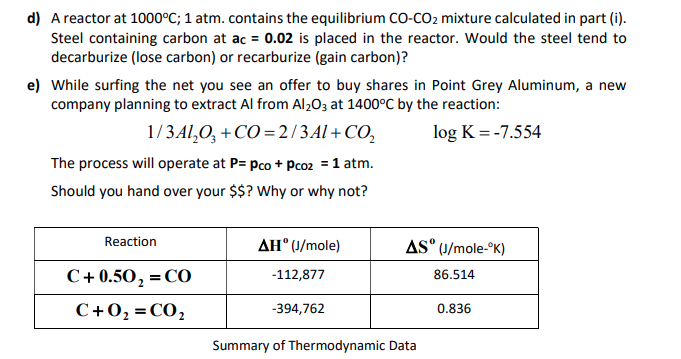








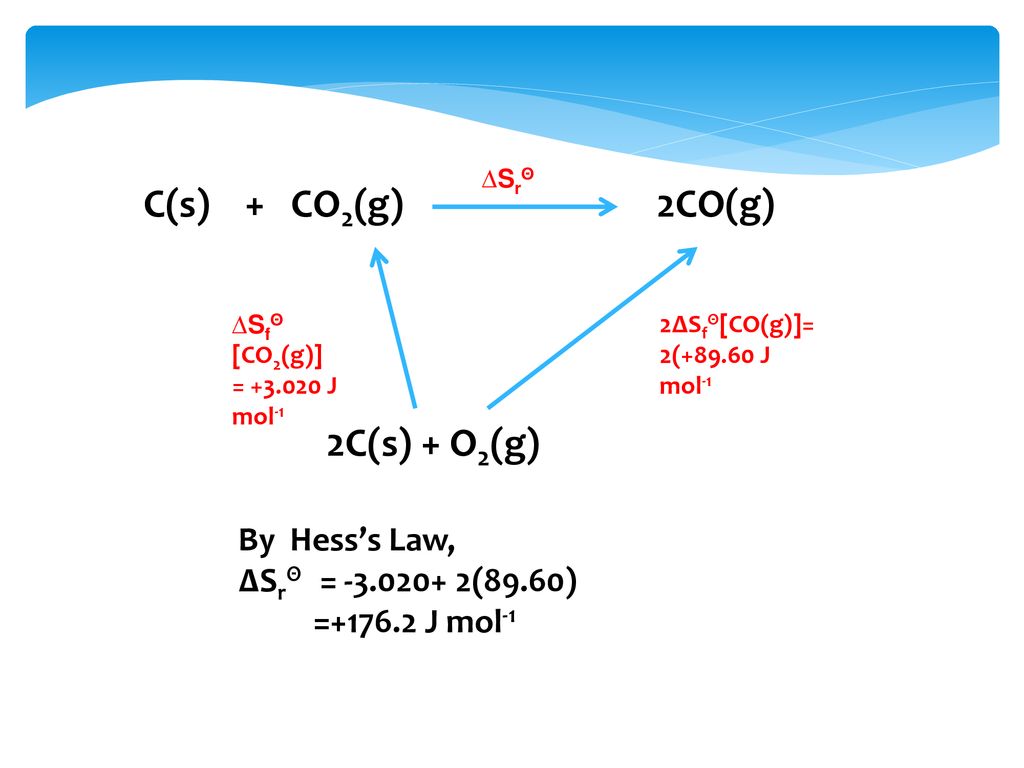

![ANSWERED] K is 7.7x10-15 for the reaction 2CO(g)=C(s... - Organic Chemistry ANSWERED] K is 7.7x10-15 for the reaction 2CO(g)=C(s... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/53142838-1659042473.5846057.jpeg)